Technological progress. Rongsheng monolithic refractory manufacturer, environmentally friendly fully automatic monolithic refractory production line, specializes in providing high-quality refractory products for high-temperature industrial furnace linings. After years of hard work, Rongsheng’s refractory products have been sold to more than 120 countries around the world, such as South Africa, Chile, Egypt, Colombia, Uzbekistan, Italy, Indonesia, Ukraine, Hungary, Spain, Kenya, Syria, Zambia, Oman, Venezuela, India, Peru, the United States, Ethiopia, Iran, Iraq, Israel, etc. Rongsheng’s iron trough castable, Al₂O₃-SiC-C castable, has also improved its oxidation resistance compared to before. Contact Rongsheng for detailed information. So, how does Al₂O₃-SiC-C castable prevent oxidation?

Preferential Oxidation of Carbon Source
Antioxidants are added to the castable material of the iron trough. The main principle is that the affinity of the antioxidant with oxygen is greater than the affinity of carbon with oxygen. The added antioxidant reacts with oxygen before carbon to achieve an anti-oxidation effect. Anti-oxidation mechanism of adding borides, Si and Al:
- (1) The additive itself undergoes oxidation reaction before carbon.
- (2) Part of the low-melting phase is generated during the high-temperature process, which blocks the porosity inside the material and increases the density of the material, resulting in the material having good anti-oxidation properties.
The effect of the amount of metal silicon powder added on the performance of the iron trough castable. From the cross-section, as the amount of metal silicon powder added increases, the area of the darker color area in the cross-section increases, indicating that the anti-oxidation effect is better. During the sintering process of the sample, the anti-oxidation mechanism of metal silicon powder as an antioxidant is as follows:
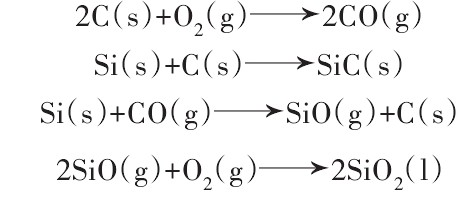
In the same time, as the amount of metal silicon powder added increases, the amount of carbon reduced in the sample increases. At the same time, the more SiO2 is generated by the reaction, of which SiO2 is in the liquid phase, which can close the pores and increase the density. In this way, the anti-oxidation performance and comprehensive mechanical properties of the castable are improved.

Adding metal Al powder to carbon-containing refractory materials can have an antioxidant effect. This is mainly because during the heat treatment process, Al reduces CO to C(s) and generates Al2O3, which inhibits the oxidation of C. The reaction formula is as follows:
![]()
Volume expansion will occur during this reaction process, increasing the density of the material structure, thereby hindering the oxidation of carbon. However, metal Al will react with carbon above 1 000°C to generate Al4C3. And Al4C3 will react with water vapor during the reaction process:
![]()
The material will have a significant volume expansion, seriously deteriorating the comprehensive performance of the material.
During the oxidation process at 1100°C and 1500°C, the antioxidant is BN, and its reaction equation is:
![]()
This shows that BN has an antioxidant effect. In addition, the generated B2O3 promotes the sintering of the sample, blocks the pores of the sample, and makes the structure of the sample denser. But the generated N2 will also cause the volume expansion of the sample.
In addition, B4C was added to Al2O3-SiC-C castable to study the effect of B4C on its mechanical properties. B4C will undergo oxidation reaction in air atmosphere:
![]()
The generated B2O3 liquid phase can promote sintering and improve the anti-oxidation effect of the material. The improvement of anti-oxidation property also further inhibits the oxidation of carbon-containing materials such as SiC, and also has a positive effect on the high-temperature flexural strength of the sample.
![]()
It can be seen that the anti-oxidation mechanism of CaB6 is the same as that of B4C and BN. They all react with O2 to generate liquid phase, and then this liquid phase further promotes the sintering of the sample. Improve the density of the material, and then improve the anti-oxidation effect of the material.
Reaction to form a protective layer
Unlike additives that preferentially oxidize carbon sources, some low-melting-point additives form a liquid phase with oxygen or components in the material at high temperatures. The generated liquid phase forms a protective layer on the surface of the material and fills the pores inside the material to prevent oxygen from entering the material, thereby achieving a certain anti-oxidation effect.
Si3N4-Fe was added to Al2O3-SiC-C castables and its effect on material properties was studied. The experiment found that when Si3N4-Fe was added to Al2O3-SiC-C castables, Si3N4 in Si3N4-Fe was first oxidized, and SiO2 was generated on the surface of the sample, which formed a low-melting phase with Al2O3 or impurities in the material, and further formed a glass phase film on the surface of the sample. It prevented O2 from entering the sample, thereby achieving a certain anti-oxidation effect.
ZrB2 was added to Al2O3-SiC-C castables. It was found that the thickness of the oxide layer gradually decreased with the increase of the amount of ZrB2 added. In the presence of carbon, carbon is first oxidized to CO, and CO continues to react with ZrB2 as follows:
![]()
On the one hand, it hinders the oxidation of carbon. On the other hand, the generated B2O3 liquid phase and the liquid phase generated by the reaction of B2O3 and CaO form a protective layer on the surface of the material. It hinders O2 from entering the interior of the sample and further prevents the oxidation of carbon.
The effects of andalusite particle size, composition and addition amount on the performance of aluminum silicon carbide refractory materials were studied. The experiment found that when andalusite was added to Al2O3-SiC-C castables, the silicon-rich glass phase generated blocked the pores inside the material due to the transformation of andalusite into mullite. In addition, the generated glass phase formed secondary mullite with the original matrix and aggregate of the material, which enhanced the dense structure of the material. Both of these help to prevent O2 from entering the material. The effect of andalusite particle size and purity on the anti-oxidation effect of Al2O3-SiC-C castables. The anti-oxidation effect of andalusite with a particle size of 1 to 3 mm is better than that of 5 to 8 mm and 3 to 5 mm. This is because the smaller the particle size of andalusite, the lower the purity, and the faster the mullite transformation rate. Based on the fact that andalusite has a certain anti-oxidation ability. Replace part of the antioxidant in the castable with andalusite. The experiment found that replacing part of the antioxidant silicon in the castable with andalusite has a better anti-oxidation effect than the antioxidant silicon powder. At present, there have been many studies on the addition of andalusite to Al2O3-SiC-C castables. The anti-oxidation effect of andalusite on castables mainly comes from the high-temperature phase transformation of andalusite to produce mullite and silicon-rich glass phase. As an aluminosilicate mineral, kyanite also has high corrosion resistance and good mechanical properties when converted into mullite after calcination. By adding kyanite powder to Al2O3-SiC-C castables, it was found that mullite phase was detected in the sample with kyanite powder added, and the formation of liquid phase reduced the apparent porosity of the sample and increased the density.
Add calcium aluminotitanate to the iron ditch castable. Replace the brown corundum in the iron ditch castable with calcium aluminotitanate, and the content of Al2O3, CaO and TiO2 in the sample increases. During the high-temperature reaction process, CaO, SiO2 and Al2O3 in the matrix react to generate low-melting phases such as CaSi2Al2O8 and mullite, which increases the density of the sample, hinders O2 from entering the material, and improves the anti-oxidation effect. However, when the substitution rate of calcium aluminate titanate is too high, the increase in the amount of low-melting phase and mullite generated causes the volume expansion of the sample, leading to the proliferation and expansion of cracks, which in turn reduces the anti-oxidation effect and mechanical properties of the sample.
Optimizing the Form of Carbon Source
Common carbon sources for iron trough castables include high-temperature modified asphalt, spherical asphalt, flake graphite, etc. Researchers are trying to find a way to modify graphite, that is, to optimize the form of carbon source to improve the anti-oxidation effect. At present, one way to optimize the form of carbon source is to add a coating on the surface of graphite so that oxygen cannot directly react with carbon. The first thing to consider when choosing a coating is whether the thermal expansion coefficient between the coating and the carbon-containing refractory material matches. The most commonly used coatings are silicon nitride and silicon carbide, which have similar thermal expansion coefficients to the carbon matrix and good chemical compatibility. According to the actual use environment of the coating, the following factors should also be considered when formulating the coating:
- (1) Diffusion rate of oxygen.
- (2) Fluidity of the coating raw material at high temperature.
- (3) Chemical stability.
The second form is to change the carbon source by adding modified graphite, such as composite carbon source form, granulated graphite, carbon black, etc. Carbon oxidation is reduced by increasing the fixed carbon content of the carbon source and reducing the carbon volatility. The effect of the amount of granulated graphite added on the properties of Al2O3-SiC-C castables was studied. Granulated graphite with a carbon mass fraction of 35% was used as the carbon source, and its effect on the anti-oxidation properties of Al2O3-SiC-C castables was investigated by changing its addition amount.
Al2O3-SiC-C castables have been widely used in castables for iron troughs, and currently have excellent use effects. However, how to further extend the service life of the material and improve the performance of the material depends to a large extent on its anti-oxidation effect. Al and Si powders will undergo hydration reactions. Although the liquid phase generated by low-melting phase additives will block the pores inside the sample and increase the density. However, excessive liquid phase causes the volume expansion and crack propagation of the sample. At the same time, the liquid phase generated by the additives during the heat treatment process will form a thin film on the surface of the material to prevent oxygen from entering the material and reduce the oxygen partial pressure inside the material. Secondly, adding a coating on the surface of the carbon source, although this achieves a certain anti-oxidation effect, there are also problems such as deterioration of material performance and cumbersome production. In order to further improve the anti-oxidation technology of Al2O3-SiC-C iron trough castables and extend the service life of iron trough castables, studying low-cost anti-oxidation technology that can simultaneously improve the anti-oxidation effect and material performance is one of its important development directions.

